RESEARCH
Research Areas
BACKGROUND
Chronic and difficult to heal wounds affect nearly 6.5 million people in the US with an excess of US$ 25 billion spent annually on its treatment Due to their associated co-morbidities such as diabetes and obesity, their true impact on the health of patients are often masked.
Current data indicate that open wounds with delayed healing are a significant risk factor for mortality from direct and indirect complication from infections. In this regards, the average survival from diabetic foot ulcers is 5 years, on par with those of common cancers Considering an ageing population, disabled veterans from multiple combats, increasing incidence of obesity and diabetes as well as poor nutrition, the incidence and the cost of treatment as well as the associated complications and mortalities will only rise in the coming years. Furthermore, the increasing prevalence of antibiotic-resistant strains of bacteria will limit the available options for treatment of infections acquired via open chronic wounds. A clearer understanding of the underlying biochemical dysregulation will allow for the initiation of multiple and mechanistically relevant healing pathways that include biochemical interventions of the chronic wound environment by directly addressing the primary roadblocks to the healing process.
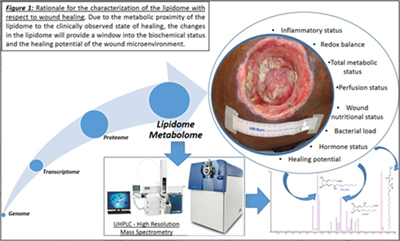
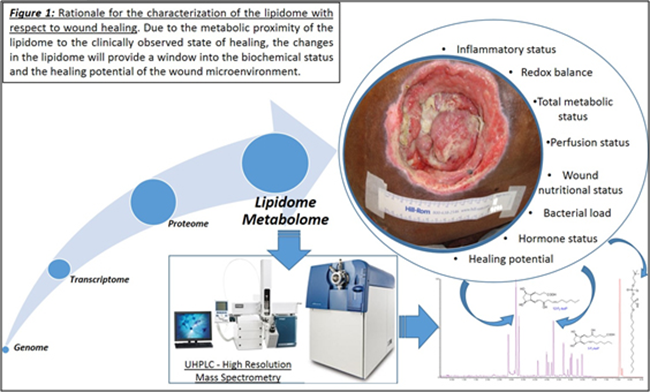
Currently, research with respect to the genome, transcriptome and the proteome have met with limited mechanistic and therapeutic success in regards to chronic wounds. These failures can be attributed to the presence of multiple redundant pathways and post-translational modifications minimizing their impact in translatability to the disease state. One underexplored avenue in the context of chronic wounds and wound healing, in general, is the lipidome.
This is despite the fact that the lipidome constitutes the most proximal of the biological molecules to the observed disease phenome and constitutes the final integrated coded and non-coded (non-enzymatic) response of the body to the wound healing process. As such, it closely reflects the biochemical status of the disease. In addition, the lipidome has demonstrated the ability to regulate the proteome and the transcriptome. Our published studies to date demonstrate that lipids are important and highly relevant regulators of the wound healing process.
RESEARCH HYPOTHESIS
Our work to date has led us to hypothesize that lipids play a significant and highly relevant role in determining the outcome of the wound healing process. Furthermore, we further hypothesize that the changes in the lipidome is diagnostic and predictive of the course and the outcome of the injury and can be used as a surrogate in identifying the appropriate treatment intervention.
HIGHLIGHTS OF CURRENT FINDINGS
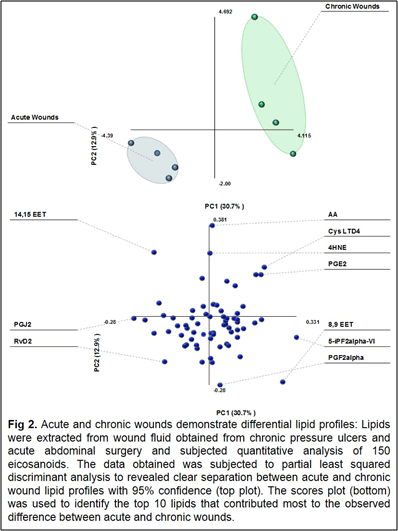
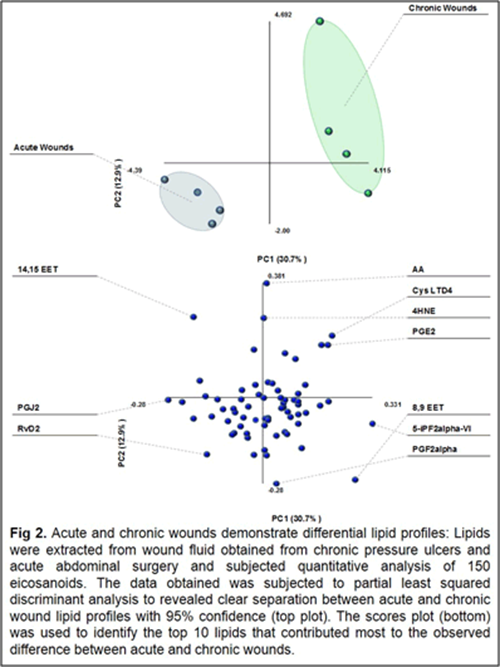
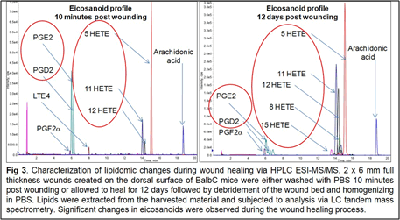
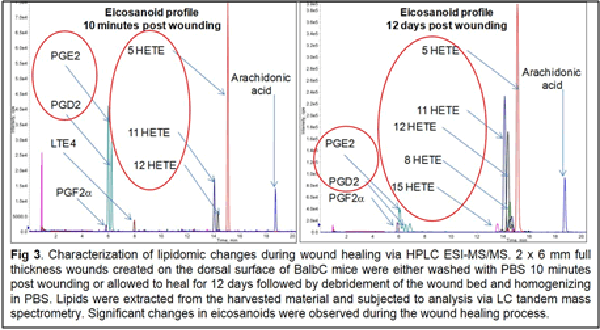
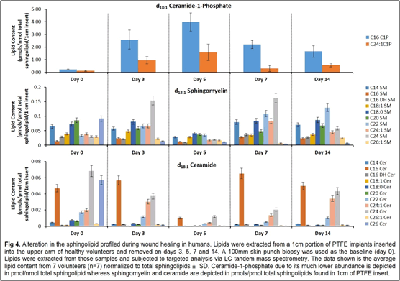
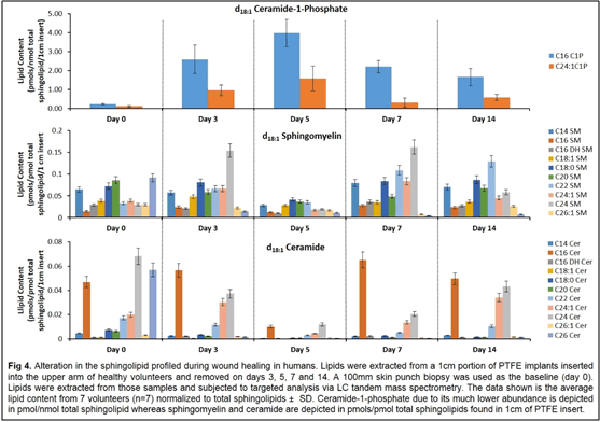
Our work to date has demonstrated the feasibility of using LC-tandem mass spectrometry to investigate the lipid-mediated signaling during the course of wound healing in humans and as well as in animal models. Furthermore, we have demonstrated for the first time a central regulatory role for sphingolipids in regulating the eicosanoid response to wounding
1. Wijesinghe, D. S. et al. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J. Lipid Res. 51, 641–651 (2010).
2. Wijesinghe, D. S. et al. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J. Lipid Res. (2014). doi:10.1194/jlr.M048207
BACKGROUND
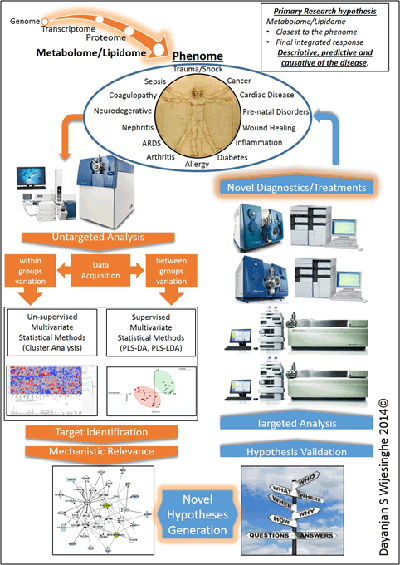
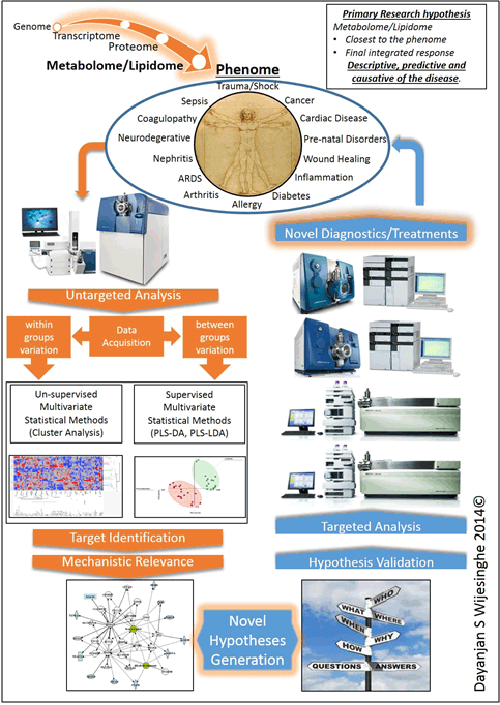
Lipidome/metabolome constitutes a highly diverse group of molecules in the body. These arise from coded biological processes (i.e. enzymatic processes), non-coded metabolic processes or via a combination of both. This diverse set of molecules constitute the final integrated response of an organism to an insult. As such they are the closest in proximity to the disease phenome and act as excellent surrogates to obtain a readout of the sum integrated responses. Significant changes will be observed in the lipidome and the metabolome prior to the onset of any clinical symptoms. Primary means of studying the lipidome/metabolome are NMR and LC tandem mass spectrometry. While NMR is easy to use it suffers from inadequate sensitivity to identify changes at very early stages.
Our laboratory utilizes a complete quantitative and qualitative analytical workflow investigating the changes of the lipidome/metabolome with respect to various disease states. Our ultimate goal is to identify pre-clinical biomarkers that will serve as molecular guide posts to direct protocolized medical interventions.
RESEARCH HYPOTHESIS
Based on our current studies, our primary hypothesis is that the lipidome and the metabolome will reflect subclinical biochemical changes to the phenome and that these changes will help identify the clinical trajectory of a patient well in advance of clinical manifestation of symptoms.
HIGHLIGHTS OF CURRENT FINDINGS
Our findings to date demonstrate the relevance of the lipidome/metabolome towards the prediction of the clinical course of cardiac arrest, sepsis, trauma and kidney disease. These findings are currently in various stages of the publication process.